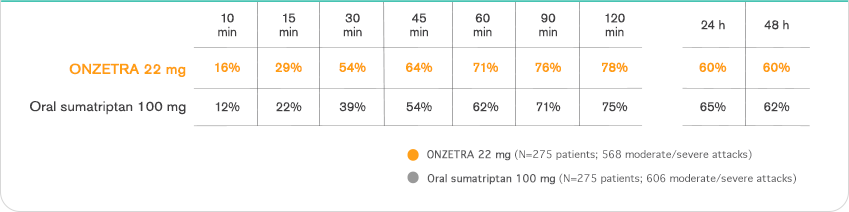
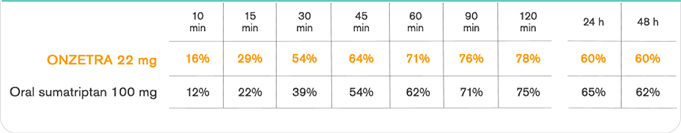
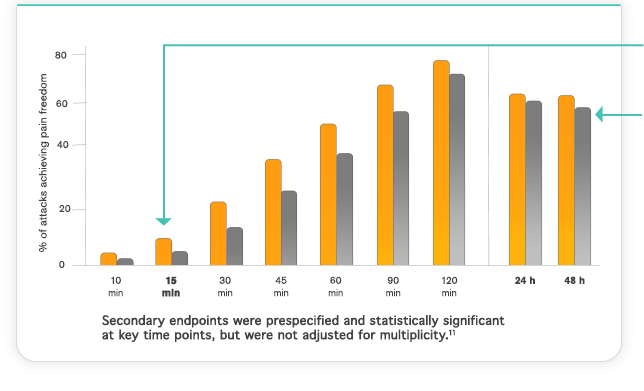
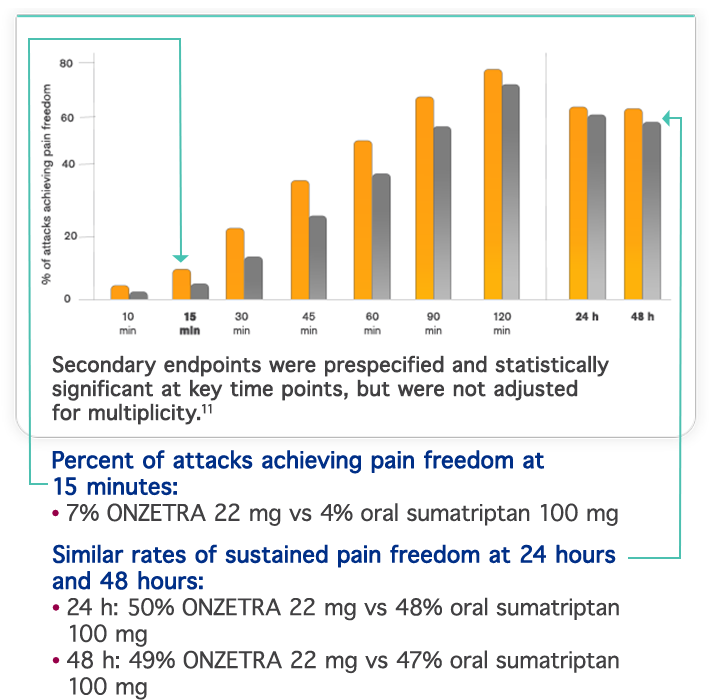
Important Safety Information & Indication
ONZETRA Xsail is contraindicated in patients with:
- Ischemic coronary artery disease (CAD) or coronary artery vasospasm, including Prinzmetal’s angina
- Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders
- History of stroke, transient ischemic attack (TIA), or hemiplegic or basilar migraine
- Peripheral vascular disease
- Ischemic bowel disease
- Uncontrolled hypertension
- Recent (i.e., within 24 hours) use of ergotamine-containing or ergot-type medication, or another 5-HT1 agonist
- Concurrent or recent (within 2 weeks) use of a MAO-A inhibitor
- Hypersensitivity to sumatriptan (angioedema and anaphylaxis seen)
- Severe hepatic impairment
WARNINGS AND PRECAUTIONS
- Myocardial ischemia/infarction, Prinzmetal's angina: These events may occur even in patients without known cardiovascular disease. Perform cardiac evaluation in triptan-naïve patients with multiple risk factors and, if satisfactory, administer first dose of ONZETRA Xsail in a medically-supervised setting
- Arrhythmias: Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1 agonists. Discontinue ONZETRA Xsail if these disturbances occur
- Sensations of chest/throat/neck/jaw pain, tightness, pressure, or heaviness: Commonly occur after treatment with 5-HT1 agonists and are usually non-cardiac in origin. Perform a cardiac evaluation in patients with cardiac risk
- Cerebrovascular Events: Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, and some have resulted in fatalities. Discontinue ONZETRA Xsail if a cerebrovascular event occurs. Before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, exclude other potentially serious neurological conditions
- Other Vasospasm Reactions: 5-HT1 agonists, including ONZETRA Xsail, may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction, splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of a vasospastic reaction following the use of any 5-HT1 agonist, rule out a vasospastic reaction before using ONZETRA Xsail
- Medication Overuse Headache: Overuse of acute migraine drugs may lead to exacerbation headache (medication overuse headache). Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms may be necessary
- Serotonin Syndrome: May occur with triptans, including ONZETRA Xsail, particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs). The onset of symptoms usually occurs within minutes to hours of receiving a new or greater dose of a serotonergic medication. Discontinue ONZETRA Xsail if serotonin syndrome is suspected
- Increases in Blood Pressure: Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported in patients treated with 5-HT1 agonists. Monitor blood pressure in patients treated with Onzetra Xsail
- Hypersensitivity Reactions: Hypersensitivity reactions, including angioedema and anaphylaxis, have occurred in patients receiving sumatriptan. Such reactions can be life threatening or fatal. ONZETRA Xsail is contraindicated in patients with a history of hypersensitivity reaction to sumatriptan
- Seizures: Seizures have been reported following administration of sumatriptan, with or without predisposing factors. ONZETRA Xsail should be used with caution in patients with a history of epilepsy or conditions associated with a lowered seizure threshold